Basophils: Basophil Function, Origin and Related Conditions
An important role for the rarest granulocyte immune cell in host defense and allergic response

Complete the form below to unlock access to ALL audio articles.
There are a group of cells dedicated to defending our bodies from all comers, these are the immune cells. Like an army, there are members that perform different tasks and that specialize in different functions, working together to overcome threats.
In this article, we consider what basophils are, how they are generated, their function in the body and medical conditions related to them.
What are basophils?
Basophils are a type of immune cell that belong to the granulocyte group along with neutrophils and eosinophils. They were first described by Paul Ehrlich in 18791 and are part of the innate immune system, providing frontline defenses against invaders such as pathogens, parasites and allergens.
They are the rarest of the granulocytes, accounting for less than 1% of all immune cells in the body, and have a distinctive, granular appearance under the microscope. This is due to the presence of cytoplasmic granules containing histamine and heparin that stain purple to blue with basic dyes such as methylene blue. The cells are 12–15 µm in diameter and have S-shaped or bi-lobed nuclei.2
Basophils start life in the bone marrow,3 as with many immune cells, as hematopoietic stem cells that are able to differentiate into numerous cell types. To form a basophil, these stem cells must first differentiate into myeloid progenitor cells followed by granulocyte-macrophage progenitors. Some of these go on to become granulocyte progenitors from which basophils then form (Figure 1). This differentiation happens in the bone marrow and basophils enter the circulation as terminally differentiated, mature cells and do not typically proliferate after maturation. The lifespan of a basophil is relatively short, typically thought to be from a few hours to less than three days.4

Figure 1: Diagram showing the differentiation pathway for the development of basophils. Credit: Technology Networks.
Basophil function
Basophils have similar effector functions to mast cells, another immune cell type, although they differ in many other ways including in their lifespan, anatomical localization and ability to proliferate. Namely, they elicit host protective or allergic responses. Unlike mast cells they are predominantly found in circulation. Basophils bind to immunoglobulin E (IgE) by a high affinity receptor5 that leads to degranulation and release of inflammatory mediators such as histamine, which promotes vasodilation, and proteases. They are also capable of secreting a number of cytokines, leukotrienes and chemokines. Other receptor-mediated stimuli, such as C3a and C5a (part of the complement system) and bacterial peptide formyl-methionyl-leucyl-phenylalanine (fMLP), can activate basophils. In addition, they can be stimulated by ionophores, lectins and allergens, which do not require specific receptors, and primed by several cytokines including, but not exclusively, interleukin 3 (IL-3), IL-5, nerve growth factor (NGF) and RANTES (regulated on activation, normal T cell expressed and secreted)6 (Figure 2).
Basophils can degranulate without necessarily undergoing cell death, releasing histamine and inflammatory mediators into the surrounding tissue. However, in some situations, basophils may be exposed to high levels of toxins, stress or certain immune responses and consequently undergo apoptosis.
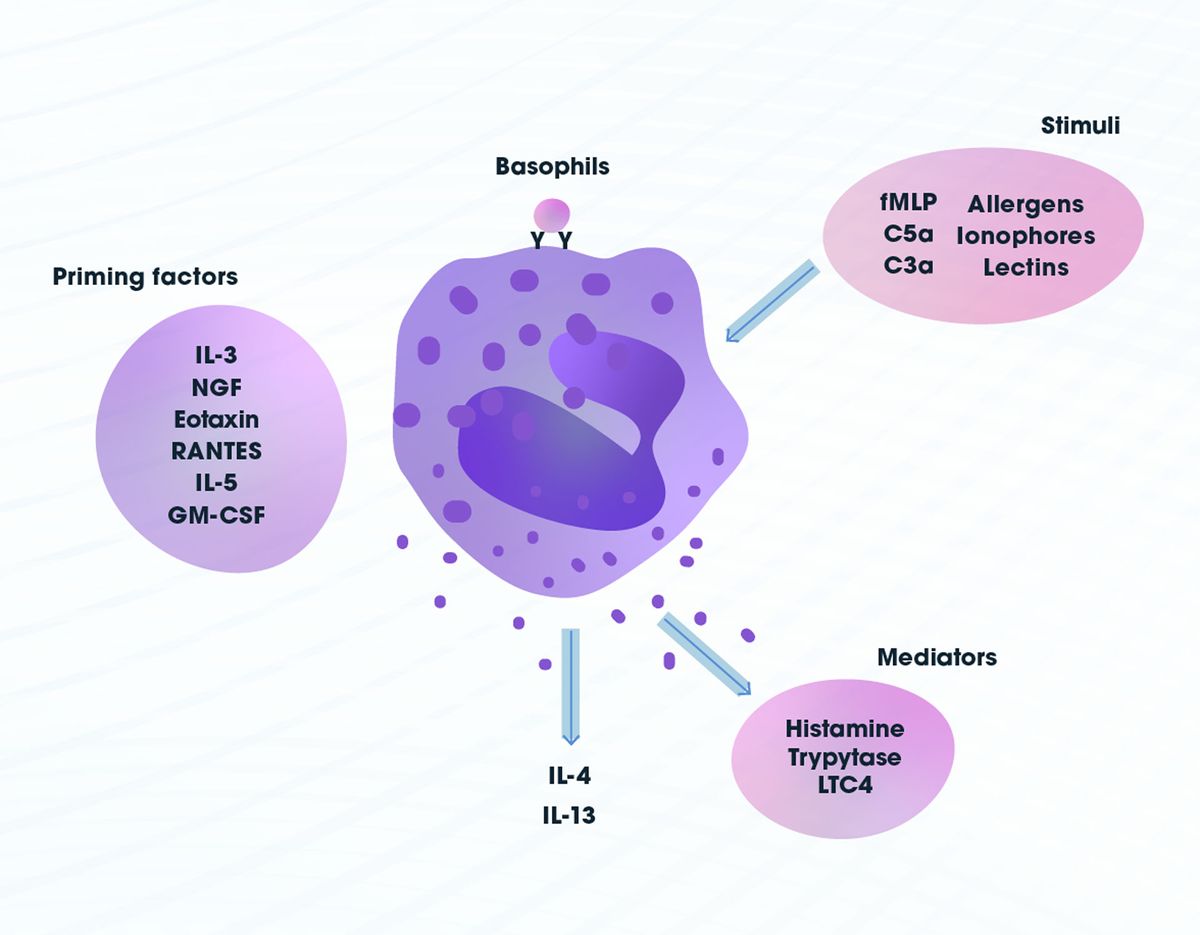
Figure 2: Basophil stimuli, priming factors and mediators. Credit: Technology Networks.
Basophils play a key role in the innate immune response to parasitic infections such as helminthic worms and they have been shown to have roles in fighting off bacterial7 and viral infections as well as venoms. Additionally, Basophils link the adaptive and innate immune responses. For example, T-cell8 and macrophage differentiation induced by IL-4 and IL-13 released by basophils assists in parasite clearance, and IL-4 release by basophils has been linked to eosinophil migration and B-cell activation.
Basophils also play a role in a wide variety of allergic and autoimmune responses. These include allergic reactions of the skin, respiratory tract and gastrointestinal tract. When the body contacts an allergen, basophils release histamine that causes the symptoms associated with conditions like hay fever. Occasionally, this can be extreme and anaphylactic reactions may occur that require immediate medical attention.
Basophil-related conditions
While not a common finding, abnormalities in the numbers of circulating basophils, be it an increase (basophilia) or decrease (basopenia) can be indicative of a variety of underlying conditions. Clinical presentations are diverse and typically reflect the underlying cause; let’s consider some of the most frequent causes.
Basophilia9
- In individuals with hypothyroidism, a condition primarily affecting older people, especially women, the thyroid gland does not produce sufficient thyroid hormones. This can affect many bodily functions including heart rate, temperature regulation, the rate at which calories are consumed and also lead to basophilia.
- Myeloproliferative neoplasms including polycythemia vera, primary myelofibrosis, essential (primary) thrombocythemia and chronic myeloid leukemia are associated with basophilia. The blood producing cells in the bone marrow reproduce excessively as a result of the neoplasms, leading to an increase in basophil levels. These conditions are caused by genetic mutation and generally lead to acute leukemia.
- Basophilia has been associated with autoimmune inflammatory diseases like lupus and inflammatory bowel disorders where they aid Th2 responses and release histamines.
Basopenia10
- In individuals with hyperthyroidism, the thyroid gland produces an excess of thyroid hormones and may be associated with increased heart rate, arrythmia, profuse sweating, sleep problems and can lead to basopenia. The most common causes include Graves’ disease, toxic multinodular goiter and thyroiditis.
- Acute sensitivity reactions are associated with a decrease in the number of basophils. Affected individuals present with clinical signs such as rash, itching and sneezing. In extreme cases, this can lead to life threatening anaphylactic reactions associated with a massive release of histamines from basophils, thus reducing their number.
- Some infections are associated with basopenia as numbers become depleted in the process of fighting off infectious agents.
Due to the low number circulating in the body, basophils have been slightly neglected in research. However, it has recently become more apparent how important they are in various conditions, from fighting infection to allergies and autoimmune diseases.
1. Ehrlich P. Über die specifischen Granulationen des Blutes. Arch Anat Physiol (Leipzig). 1879. 571–579.
2. Siraganian RP. Basophils. In: Delves PJ, IM Roitt eds. Encyclopedia of Immunology (Second Edition). Academic Press. 1998:332-334. doi:10.1006/rwei.1999.0086
3. Arinobu Y, Iwasaki H and Akashi K Origin of basophils and mast cells. Allergol Int. 2009 Mar;58(1):21-8. doi:10.2332/allergolint.08-RAI-0067
4. Min B, Brown MA and LeGros G. Understanding the roles of basophils: breaking dawn. Immunology. 2012 Mar; 135(3): 192–197. doi:10.1111/j.1365-2567.2011.03530.x
5. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010 Feb;125(2 Suppl 2):S73-80. doi:10.1016/j.jaci.2009.11.017
6. Schroeder JT, Chichester KL and Bieneman AP. Human basophils secrete IL-3: Evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol. 2009 Feb 15; 182(4): 2432–2438. doi:10.4049/jimmunol.0801782
7. Piliponsky AM, Shubin NJ, Lahiri AK et al. Basophil-derived tumor necrosis factor can enhance survival in a sepsis model in mice. Nat Immunol 20, 129–140 (2019). doi:10.1038/s41590-018-0288-7
8. Henry EK, Inclan-Rico JM and Siracusa MC. Type 2 cytokine responses: regulating immunity to helminth parasites and allergic inflammation. Curr Pharmacol Rep. 2017 Dec;3(6):346-359. doi:10.1007/s40495-017-0114-1
9. Naeim F, Rao PN, Song SX and Phan RT. Chapter 63 - Granulocytic disorders. In: Atlas of Hematopathology (Second Edition). Morphology, Immunophenotype, Cytogenetics, and Molecular Approaches. Academic Press. 2018:871-884. doi:10.1016/B978-0-12-809843-1.00063-2
10. Shah H, Eisenbarth S, Tormey CA and Siddon AJ. Behind the scenes with basophils: an emerging therapeutic target. Immunother Adv. 2021 Jan; 1(1): ltab008. Published online 2021 May 19. doi:10.1093/immadv/ltab008